Arthur
M Harrisson Ltd.
Cement
Raw Materials – Chalk
Contact
Home Background
Raw Materials Clinker Microscopy Secondary Raw
Materials Limestone
Cement Chemistry
Quality
Solutions
The
growth of Portland Cement manufacture, as patented by Joseph Aspdin,
largely
took place in the South and East of England during the 19th
and
early 20th Centuries.
The raw
materials available were a soft, generally saturated limestone, known
as Chalk,
and a soft largely waterlogged clay.
It
is debatable whether these circumstances enhanced the development of
Portland
Cement manufacturing technology or perhaps held it back by several
decades.
The Chalk
of Southern England is a soft, white, very
fine grained limestone
composed largely
of minute fossils known as coccoliths.
These creatures lived in ancient oceans in the Cretaceous
Period of
Geological history some 60 to 95 million years ago, roughly coincident
with the
demise of the dinosaurs. Similar
creatures to those which formed the ancient Chalk still dwell in modern oceans,
but the Cretaceous was the height of their rock-building activities. When the creatures died
and fell to the ocean
floor they were compressed over millions of years by the addition of
further
debris from above. As
well as the
calcium carbonate which formed the bulk of the skeletal material there
were
some creatures which were built on a silica framework.
The silica also fell to the bottom of the
ocean and under the pressure of the overlying sediments was
redistributed to
form nodules within the mainly limestone rock.
The
nodules are known today as flint.
oceans,
but the Cretaceous was the height of their rock-building activities. When the creatures died
and fell to the ocean
floor they were compressed over millions of years by the addition of
further
debris from above. As
well as the
calcium carbonate which formed the bulk of the skeletal material there
were
some creatures which were built on a silica framework.
The silica also fell to the bottom of the
ocean and under the pressure of the overlying sediments was
redistributed to
form nodules within the mainly limestone rock.
The
nodules are known today as flint.
Figure 1. A
coccolith
from chalk in the electron microscope.
Fig.
1 shows a typical fossil coccolith from the
chalk. Much of the
chalk is made up of
many millions of tiny
skeletons such as these. The individual plates which form the
fossil are of
the order of
one
micron across and the fragility of the structure when washed in a
washmill or a
washdrum means that a large proportion of the fragments of chalk in the
slurry
are one micron or less in size with no further milling required.
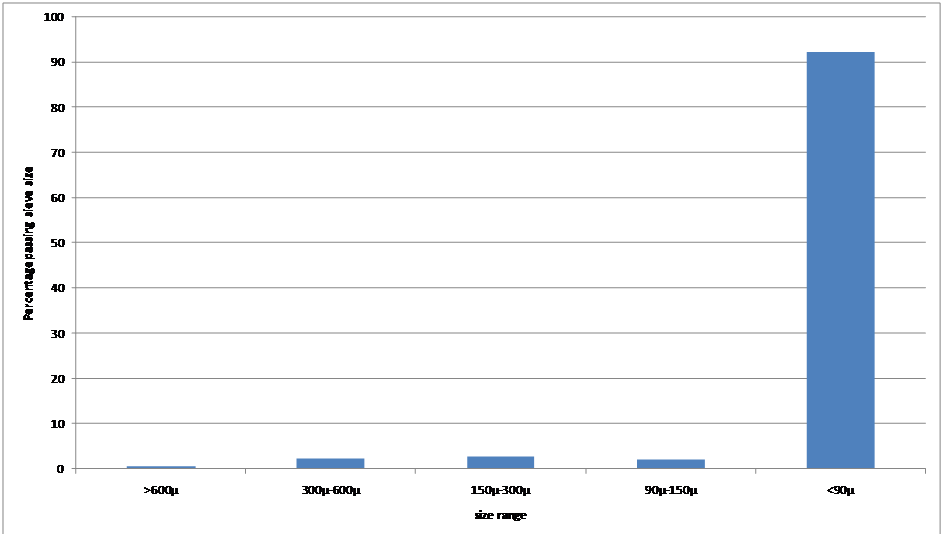
The size distribution in Fig. 2 is typical of
a chalk slurry and shows that over 90% of the chalk passes a 90µ sieve
with no grinding other than being agitated in water and the autogenous
action of
any
flint component. The
harder silica of
the flint almost certainly makes up the bulk of the 90µ+ material.
Portland
cement clinker production grew from the burning of limestone to produce
quicklime, a process which has been taking place since
ancient times.
The
Roman architect, Marcus
Vitruvius Pollio,
wrote a series of books “de Architectura”
in which he described the construction practices of his
day.
He sets out
preferences for which type of
stone is best for which purposes.
Fig. 2 Typical particle size distribution of chalk slurry.
It is
clear that in his view the best stone to make lime for building was
regarded as
thick and hard (“spisso
et duriore”). These
limestones could be more easily stacked
and burned in lime kilns until calcination was complete at relatively
low
temperatures.
The
burning of impure limestones led to the use of hydraulic lime in
construction, that is a lime with a proportion of calcium silicates and
calcium
aluminates as well as calcium oxide.
The
silicates and aluminates were produced by the reaction of calcined lime
with
clay minerals at temperatures above about 900°C.
These limes were slaked in the same way as
quicklime for use, but care needed to be taken to avoid hydration of
the other
phases in the material before it was used in practice.
Modern
producers of cement clinker will be aware
that the combination of calcium oxide with silica, alumina and iron
oxide
requires higher temperatures than lime burning but also, more
crucially, it
requires that the raw materials are extremely fine before being placed
in the
cement kiln so that the reactions can take place on a very small scale
between
grains of different composition. When firing an impure limestone the
clay may
be evenly distributed within the stone and already
able to react with the lime without the
necessity for extreme size reduction.
There are some cement raw materials which contain the
appropriate
mixture of limestone and clay to allow burning with little or no extra
effort
or addition, however, in the vast majority of cases two (or more)
components
must be brought together from different locations and the required
chemical
reactions need to be facilitated by making the individual constituents
as fine
as possible.
It
may, therefore, have been a happy coincidence
that the materials being used by Aspdin and also James Frost, James
Parker and
others to produce various hydraulic limes and cements were in a state
which
permitted mixing on a very intimate level with relatively little
physical
effort. A key
element of the various
patents in the nineteenth century was that the raw materials were
finely ground
before being fired. Joseph
Aspdin’s
patent for Portland Cement related to Carboniferous Limestones of the
Pennines
and required that the material be “puddled” or “powdered” before use. When his son William, as
well as the other
Kentish cement producers, established cement manufacturing facilities
using
soft chalks and clays, all that was required for size reduction and
blending of
the various components was washing to create a slurry from which coarse
material dropped out, leaving only the ideal sized materials for cement
clinkering reactions to occur.
It
was perhaps the relative ease of working these
materials which led to the development of what we now call the wet
method of
cement clinker manufacture. This
involves mixing the materials with water and making a slurry which goes
into a
long rotary kiln to be dried, calcined and combined to make cement
clinker. 
Based
on the wet process kilns of Southern England,
similar kilns were constructed around the world.
Where the raw materials were harder
limestone, the stone was crushed and ground, mixed with water and made
into a
slurry before being put into the wet process kiln, in which the water
needed to
be evaporated before any calcining or clinkering could take place.
For
the wet process the size reduction of the chalk and
clay by washing takes place in one of two milling systems. These are known as the
washmill and the
washdrum. The
washmill is a circular
tank into which the chalk is loaded then mixed with water. The comminution of the
chalk is achieved by
the movement of a
series of harrows
through the mix rotating around the centre of the tank, producing a
slurry of
about 40% moisture content.
The
washdrum
is similar to a ball mill without the steel media.
The action of the chalk with water while the
drum is rotated is sufficient to break down the structure of the chalk
components. In some
chalk deposits, as
described above, there are nodules of silica which are appreciably
harder than
the chalk. These
assist in the grinding
process by acting as autogenous grinding media.
The
water needs at some stage to be lost from the
slurry, in the final event by evaporation in the cement kiln. As this is expensive of
energy, any reduction
in the water content before the kiln is advantageous. This can
sometimes be
achieved by the use of water reducing additives but while these may be
successful with the purer chalks, any clay content may react adversely
with the
additives making the slurry more, rather than less, viscous when energy
is
applied to move it.
The
energy requirements using chalk
based slurry are
evidently very high by modern standards and it was the drive to reduce
these that
led to the investigation of other methods of size reduction and
minimising the
heat used in evaporation in times when all fuels were won from mining
or
drilling and the costs of production were directly related to the use
of fuel,
before the arrival of alternative fuels with negative or minimal costs.
The
development of more efficient dry process kiln
systems with preheaters and precalciners did
not, however, solve the problem
that the chalk (and usually also the clay component of the mix) are
taken from
the ground frequently with over 20% moisture and are not easily milled
in
conventional ball mills or vertical roller mills.
An early solution to the problem of poor
efficiency of wet kilns was to put the slurry through a drying process
before
the kiln. A common
practice in the
1970s and 1980s was
to place a filter
press between the raw material preparation and the cement kiln. This provides the
opportunity to reduce the
moisture from the slurry (about 36-40% moisture) to about 18% moisture
out of
the filter press. The
filter cake can be
fed into a semi-wet Lepol type kiln, where drying takes place on a
moving
grate, or directly into a shortened wet process kiln.
through a drying process
before
the kiln. A common
practice in the
1970s and 1980s was
to place a filter
press between the raw material preparation and the cement kiln. This provides the
opportunity to reduce the
moisture from the slurry (about 36-40% moisture) to about 18% moisture
out of
the filter press. The
filter cake can be
fed into a semi-wet Lepol type kiln, where drying takes place on a
moving
grate, or directly into a shortened wet process kiln.
While
this achieved some level of success the use of
filter presses is, itself, of poor efficiency and the use of
electricity to
operate the presses is generally outside the control of the cement
plant. The advent
of alternative fuels does not
encourage the use of such energy.
The
persistence of washmills is therefore still a feature of relatively new
cement
plants using these materials, such as those at Rugby in England and
Aalborg in
Denmark, even though the slurry is then to be fed into a dry process
cement
kiln. The
development of the flash drier
in a semi-wet kiln system has allowed these processes to be combined
while
still maintaining relatively efficient combination at about 1000
kcals/kg
clinker.
A
further development of this use of chalk in cement
making has been accomplished at Chelm in Eastern Poland where a 5000
tpd cement
kiln operates with no raw material grinding at all.
Chalk and Marl are dug from the quarries
then, after a primary crusher, they are stockpiled in linear stacker
reclaimer
sheds. The
materials are put through hoppers
and fed directly into a drier crusher at the base of the preheater
tower. Waste gases
from the kiln pass through the
chamber where a giant hammer mill breaks up the agglomerates from the
chalk and
marl and the fines are carried to the top of the tower from where they
progress
through a conventional dry process kiln with precalciner. The need to maintain
enough heat to dry the
materials limits the number of cyclones in such a kiln to two or three,
but the
fuel consumption can be as low as 800 kcals/kg.
These
developments are a result of the ability to
use water as the medium for size reduction of the chalk raw material. With harder raw materials
the size reduction
is of necessity in a raw mill and the use of the wet process seems
today
totally irrational for designing a cement plant.
There are, however, a considerable number of
cement plants around the world where materials have been won from the
ground
with a moisture component, put through a raw mill where they are dried,
then
more water is added to make a slurry after which the water is
evaporated in the
long wet cement kiln. It
seems quite
likely that, had William Aspdin not left his native Northern English
home with
its Carboniferous Limestone and had he continued to make his
revolutionary cement
clinker with its integral flux phase, he or his successors may have
worked out
a more efficient way to use the scarce fuel resources a little sooner
than in
practice was the case.
If
the early cement manufacturers had concentrated
more on the development of the early shaft kilns, modified into “bottle
kilns”
to increase draft and therefore temperatures, with the efficient heat
transfer
characteristic of this type rather than developing a system for
evaporating
water, then it may be that a dry process would have developed earlier,
with the
rotary kiln being an add-on to achieve better clinkering.
A
Harrisson 2011
 oceans,
but the Cretaceous was the height of their rock-building activities. When the creatures died
and fell to the ocean
floor they were compressed over millions of years by the addition of
further
debris from above. As
well as the
calcium carbonate which formed the bulk of the skeletal material there
were
some creatures which were built on a silica framework.
The silica also fell to the bottom of the
ocean and under the pressure of the overlying sediments was
redistributed to
form nodules within the mainly limestone rock.
The
nodules are known today as flint.
oceans,
but the Cretaceous was the height of their rock-building activities. When the creatures died
and fell to the ocean
floor they were compressed over millions of years by the addition of
further
debris from above. As
well as the
calcium carbonate which formed the bulk of the skeletal material there
were
some creatures which were built on a silica framework.
The silica also fell to the bottom of the
ocean and under the pressure of the overlying sediments was
redistributed to
form nodules within the mainly limestone rock.
The
nodules are known today as flint. 

