From my laboratory in North Wales I can offer assessments of clinker samples including:


Each cubic millimetre of solid clinker can easily
contain
over 8000 crystals, frequently very many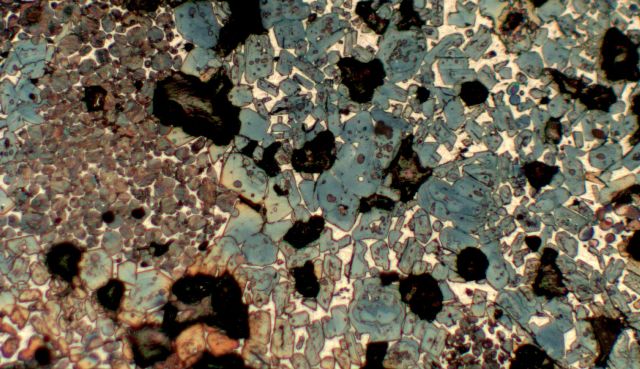 more.
By slicing open a clinker nodule before it is ground up to
make cement
and polishing the surface to
a very high degree we are able to see the crystals
under the microscope. By
staining or
etching the surface we can make the different types of crystal show up
as
different colours and so have a slice
of the internal structure of the clinker,
frozen as it left the burning zone at about 1450°C and fell into the
cooler.
Figure 1. Polished section of clinker
etched with
nital.
more.
By slicing open a clinker nodule before it is ground up to
make cement
and polishing the surface to
a very high degree we are able to see the crystals
under the microscope. By
staining or
etching the surface we can make the different types of crystal show up
as
different colours and so have a slice
of the internal structure of the clinker,
frozen as it left the burning zone at about 1450°C and fell into the
cooler.
Figure 1. Polished section of clinker
etched with
nital.
A considerable amount can be gleaned from the clinker microstructure about how the raw feed was prepared, how the temperature profile in the kiln affected the crystal growth, how quickly the clinker was cooled by secondary air after leaving the burning zone and before falling into the cooler and even about how the cement made from this clinker is likely to behave in service.
The photomicrograph above shows a polished section of a clinker nodule etched with a mixture of alcohol and nitric acid. This causes the alite crystals in the clinker to turn blue and the belite crystals brown when viewed in reflected light under the microscope.
From the relative sizes and shapes of the crystals, from their positions in relation to each other, from any inclusions within the crystals, from the nature of the interstitial material, even from the shapes of the pores formed within the clinker and filled with polishing debris during preparation, we can put together the story of the clinker’s origins and future as well as some indications of the degree of efficiency in how the kiln is being burned.
The
blue, angular crystals in the picture are of alite,
tricalcium silicate. These are generally
described as angular, hexagonal crystals, commonly approximately 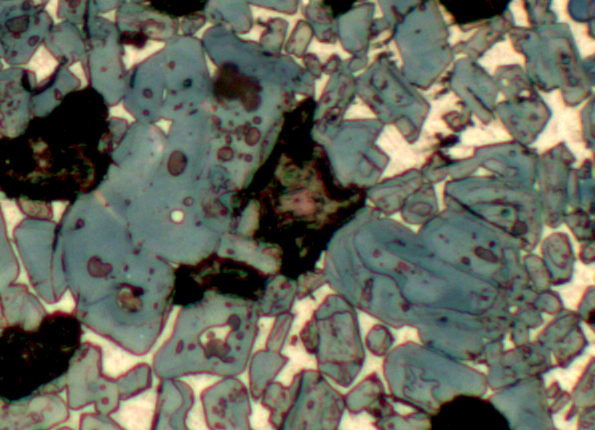 30-35 microns
across in section. They
comprise typically
between 50 and 70 percent of the cement clinker, sometimes more or less
than
this. The reaction
of these crystals
with water is crucial to the development of strength when mortar or
concrete
are mixed and placed. The
size, shape and
composition of alite crystals is dependent
on the chemistry of the raw mix, the fineness of the kiln feed, the
maximum
temperature experienced by the clinker during its formation in the
cement kiln
and the length of time at high temperature.
30-35 microns
across in section. They
comprise typically
between 50 and 70 percent of the cement clinker, sometimes more or less
than
this. The reaction
of these crystals
with water is crucial to the development of strength when mortar or
concrete
are mixed and placed. The
size, shape and
composition of alite crystals is dependent
on the chemistry of the raw mix, the fineness of the kiln feed, the
maximum
temperature experienced by the clinker during its formation in the
cement kiln
and the length of time at high temperature.
The crystals in the picture are very varied in size. The examples in the top right corner of the detail (Fig. 2) are not much over 25 microns in length. They are angular in shape (euhedral) and separated in the matrix, formed by the crystallisation of the liquid phase of the clinker. The larger crystals in the centre of Fig. 2 appear to be agglomerations of smaller crystals, each of them larger than the first set of crystals at about 35 to 40 microns in length, with overall length of the combined crystals approaching 80 microns. The shape of the crystals is less clearly hexagonal and can be described as subhedral. These large composite crystals contain large numbers of rounded inclusions of brown belite crystals. As the smaller alite crystals coalesced to form the larger crystals some belite became included as lime was unable to reach the belite and convert it to alite. Although there are a small number of inclusions of white liquid phase, trapped within the alite as the smaller crystals coalesced, these are rare and the local shortage of flux contributed to the inability to convert the belite inclusions to alite. At the bottom of Fig. 2 the alite crystals are finer again, but poor in shape with rounded corners. These crystals have partly coalesced and included portions of belite and liquid phase.
The larger crystals with belite inclusions are typical of those formed from conversion of belite clusters due to the presence of coarse silica grains in the raw feed. These form from coarse belite crystals frequently surrounding a central pore and if several such clusters are present this would be evidence that the siliceous component of the kiln feed was insufficiently finely ground. The smaller alite crystals close to the coarser examples are also typical of this situation because the excessive growth of the alite is a local phenomenon brought about by the pre-growth of coarse belite, alite crystals formed by other means are not similarly affected. If the size were due to excessive overburning due to poor heat distribution in the cement kiln all the alite crystals would be expected to be large, which is generally ascribed to slow burning in a long flame. Quick burning in a short flame produces smaller alite crystals which are considered to be more reactive. In a slow burned clinker crystal growth by cannibalism is not usually found but in this example the large crystals appear to have formed from the amalgamation of several crystals by this process.
The shape of the smaller alite crystals is also of interest. Those at the top right of Fig. 2 are well separated and euhedral. Those at the bottom left are of less well formed shape and are tending to merge together. The former situation is typical of type M3 alite while the latter are more like the M1 alite variety. The difference has been described as being due to the impurities present in the clinker, primarily the balance between sulphate and magnesia. It was known that the raw feed which produced this clinker contained some magnesia and was fired using a high sulphur fuel. Having seen these differences within one field of view, the microscopist would be investigating other possible indicators of poor homogenisation of the raw feed components, leading to local preponderances of either SO3 or magnesia relative to each other. The relative proportions of each of these types of alite and the different sized crystals will have implications for the potential quality of the clinker because finer, M1 type crystals are sometimes considered more reactive than coarser or type M3 crystals.
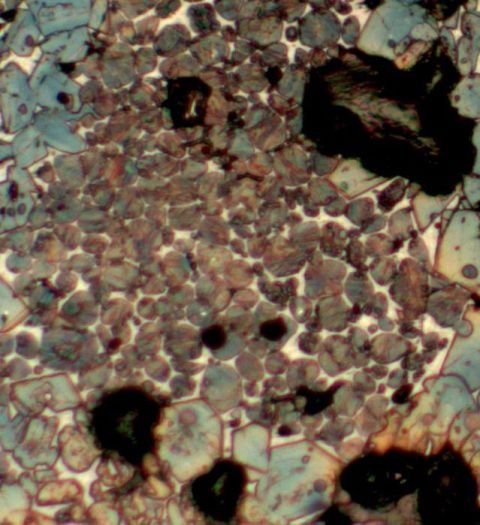 On
the left side of the main picture is a cluster of rounded
crystals, generally brownish in colour but quite variegated and with a
tendency
to a lamellar appearance. These
are
belite crystals and Fig 3 shows the cluster to contain some light
coloured interstitial
liquid phase although the crystals are quite tightly packed. In a coal fired kiln this
might be thought to
be the result of coal ash from insufficiently ground coal falling on
the
clinker bed and not being able to combine with free lime in the short
journey
through the hottest part of the burning zone.
In a kiln fired by lower ash fuels the cluster will have
come from
poorly ground raw materials, particularly shales or sands with a high
feldspar
content.
On
the left side of the main picture is a cluster of rounded
crystals, generally brownish in colour but quite variegated and with a
tendency
to a lamellar appearance. These
are
belite crystals and Fig 3 shows the cluster to contain some light
coloured interstitial
liquid phase although the crystals are quite tightly packed. In a coal fired kiln this
might be thought to
be the result of coal ash from insufficiently ground coal falling on
the
clinker bed and not being able to combine with free lime in the short
journey
through the hottest part of the burning zone.
In a kiln fired by lower ash fuels the cluster will have
come from
poorly ground raw materials, particularly shales or sands with a high
feldspar
content.
The lamellar structure is the result of a phase transition from α to α-prime on cooling. The origin and exact development of the lamellae and even their nature is a matter of debate still, but certainly they indicate that the crystal growth occurred at high temperature and the relatively small size of the crystals, averaging 15-20 microns, indicates that the high temperature was experienced for a relatively short time because these α belite crystals grow rapidly once formed.
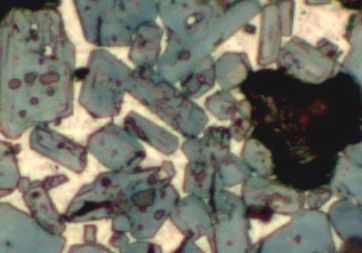
Where the matrix is more abundant the fine grey and white crystals of tricalcium aluminate and tetracalcium alumino-ferrite respectively are seen (Fig. 4). The size of these crystals indicates a relatively fast cooling rate between the hottest part of the burning zone and the kiln exit. The relative quantities of each of the crystal types suggests a typical alumina to iron ratio for a normal Portland cement (i.e. not a white cement and not a sulphate resisting cement).
Figure 4. Detail showing matrix.Putting all of these observations together and even with no further knowledge of the cement plant or raw materials a certain number of suggestions can be made as to the operating parameters of the kiln, the efficiency of the raw feed milling and homogenisation and the potential properties of the clinker in cement.
With regard to raw feed preparation the presence of a siliceous component which is difficult to reduce in size is betrayed by the presence of coarse alite crystals, associated with pores and including belite crystals. Evidently the extent of this characteristic and whether it may be problematic in free lime reduction will be explored by examination of further fields of view from this clinker nodule and from others from the same clinker sample.
The variability in shape of the smaller alite crystals suggests poor homogenisation of the raw feed and possibly poor assimilation of components introduced from the fuel. Irregular distribution of magnesium may be due to poor blending of a dolomitic portion of the limestone quarry with the purer limestone. Again, of course, the rest of this clinker sample needs to be examined to determine whether this is an isolated occurrence or a potentially significant issue.
Belite clusters indicate poor distribution of the siliceous component of the raw materials. In the case of the coarser silica grains the presence of sufficient free lime to convert belite to alite is indicated in this particular field of view. The cluster of fine belite crystals has not, however, been converted despite the presence of the liquid phase to transport dissolved lime. There is no free lime apparent in this picture and it will be necessary to examine the rest of the clinker to determine the amount of free lime which might be available. It would be a reasonable starting point to assume that free lime was not available because the lime saturation of the clinker, whether locally or generally, was low and the free lime was not present. The discovery of further free lime elsewhere in the clinker, particularly if in clusters, would cause this view to be revised and would point to further homogeneity issues with the kiln feed. Thus the process of examination will inevitably lead to revised opinions as to the state of the whole process until reasonably firm conclusions are reached.
The fineness of the liquid phases points to a moderately fast cooling rate. This, combined with the generally fine alite crystals, except in the exceptional circumstances of the coarse silica grains, and the fine α formation of the crystals indicates a well formed flame with a short relatively hot profile. In the case of a feed with a relatively low lime saturation factor, perhaps less than 95, the clinker can form with low free lime and moderate performance. If improvements in strength are required, however, simply increasing either the saturation or the silica ratio will inevitably lead to difficulty in reducing free lime and a more radical improvement in the raw feed preparation would be required.
Evidently it would be unwise to form a diagnosis of a cement plant performance from the examination of a single picture of a clinker section. It is also evident that even this small fragment leads to an indication of the nature of the clinker and with the further examination of the rest of the sample the questions which need to be asked about feed preparation and mix design will become apparent.
A Harrisson 2011