Arthur
M Harrisson Ltd.
Secondary
Raw Materials
Contact
Home
Background
Limestone
Chalk
Clinker Microscopy
Raw
Materials Cement Chemistry
Quality
Solutions
The
primary raw material for cement clinker manufacture is
limestone which provides the kiln feed with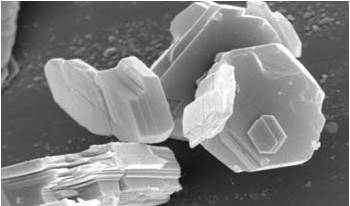 calcium
carbonate. Calcium carbonate is
calcined to remove CO2,
leaving free CaO at high temperature and in a highly reactive state to
combine
with silica and form calcium silicates, which provide the strength
potential in
portland cement. As
well as calcium
silicates the
CaO combines with aluminium oxide and iron oxide to form
a melt
which facilitates the combination of CaO and SiO2
in the kiln. The
liquid then cools as it passes the
hottest part of the kiln to crystallise out as calcium aluminates and
calcium alumino-ferrites.
calcium
carbonate. Calcium carbonate is
calcined to remove CO2,
leaving free CaO at high temperature and in a highly reactive state to
combine
with silica and form calcium silicates, which provide the strength
potential in
portland cement. As
well as calcium
silicates the
CaO combines with aluminium oxide and iron oxide to form
a melt
which facilitates the combination of CaO and SiO2
in the kiln. The
liquid then cools as it passes the
hottest part of the kiln to crystallise out as calcium aluminates and
calcium alumino-ferrites.
Fig.
1 Kaolinite in the
Scanning Electron Microscope
Limestone
therefore requires the presence of a further raw
material to provide aluminium and iron oxides as well as silica which
are
required for the process. In
some cases
these oxides can all be found in the appropriate proportions in a
single second
raw material and cement clinker can be produced from a simple two
component
mix. Because of the
inherent variability
of naturally won raw materials however, the ability to control all of
the
chemical requirements with two components is limited.
With two components the lime saturation
factor of the mix can be controlled and made consistent by making
regular
analyses of the mix and varying the proportions of the two materials. However, making these
changes to accommodate
the natural variability of one or other or both of the raw materials
will inevitably
create wider variations in the other chemical parameters used in
clinker
making, the silica ratio and the alumina to iron ratio.
Generally the
main source of silica,
alumina and iron oxides
has been clay or shale with control of the silica and alumina to iron
ratio
being accomplished by the addition of small quantities of sand and
oxide wastes
from steel manufacture.
In many cases
there is little
realistic choice of second raw
material. The
economics of transporting
bulky materials over large distances usually means that the closest
suitable
material is the one which is used.
Frequently, therefore, the ideal raw material is not
economically viable
and a compromise is made with the choice of clay, shale, marl or other
alumino-silicate deposit. In
some cases
the aluminium oxide can be supplied from a by-product source and this
will be
discussed later, but in nearly every case there will be a need for a
material
extracted from a quarry.
Clays and
shales share essentially
the same genesis, having
been formed by chemical weathering of minerals formed initially as
igneous rocks,
that is crystallised from a reservoir of molten rock either deep within
the
earth and subsequently exposed by wearing down of the surface or as
outpourings
of lava from volcanoes. The
components
of these igneous rocks vary depending on their own origin but are
variations on
a mix of quartz, feldspars (predominantly alumino-silicate crystals but
with
alkali ) micas and other minerals containing iron, magnesium and
alkalis. In
general igneous rocks contain very little sulphur, although clays and
shales
frequently have appreciable quantities of these which can sometimes
cause
problems when used as cement raw material.
The chemical
weathering of the
igneous rocks (or more commonly
rock fragments produced by mechanical weathering of the landscape by
water and
ice movement and by stresses caused by thermal expansion and
contraction)
breaks down the feldspars which are reformed as clay minerals. These are sheet structures
held together by
layers of water. Some
clay minerals
possess the property to absorb large amounts of water, swelling in the
process. This
may present significant
problems with handling the materials when introduced to a cement plant.
Mineralogically
the simplest form of
clay raw material is
one derived from weathered granite rocks and recovered in situ by
removal from
faces of granite with high pressure water jets.
The clay is removed from the parent rock along with sand,
unweathered
feldspars, micas and other minor components and is washed down slopes
of low
gradient so that successively finer material settles out with distance
from the
working face. Eventually
all that is
left in suspension is the very fine clay fraction, in this case
kaolinite clay,
which is recovered from settling ponds.
Historically the primary use for this very pure clay is
for fine china
production and it is known as china clay.
It also has a use however in the production of white
cement clinker
because of it’s purity and absence of colouring elements such as iron.
Pressure on
the clay minerals due to
the laying down of very
large volumes of the deposit over long periods of time compresses the
clay
minerals to the extent that the rock structure may become massive
rather than
plastic and may break along joint planes.
These materials are generally known as mudstones.
When the
weathering of the igneous
rocks is less complete
and fine silt sized particles of quartz and micas remain in the clay,
compression can create a fissile rock known as shale.
In cement manufacturing there is a tendency
to call all the materials, “Clays” whether fully weathered and
compressed or
not.
The nature of
the raw material has an
effect on the
burnability of the feed as well as on the chemistry of the
recirculating load
in the kiln and the final clinker.
The
presence of unweathered igneous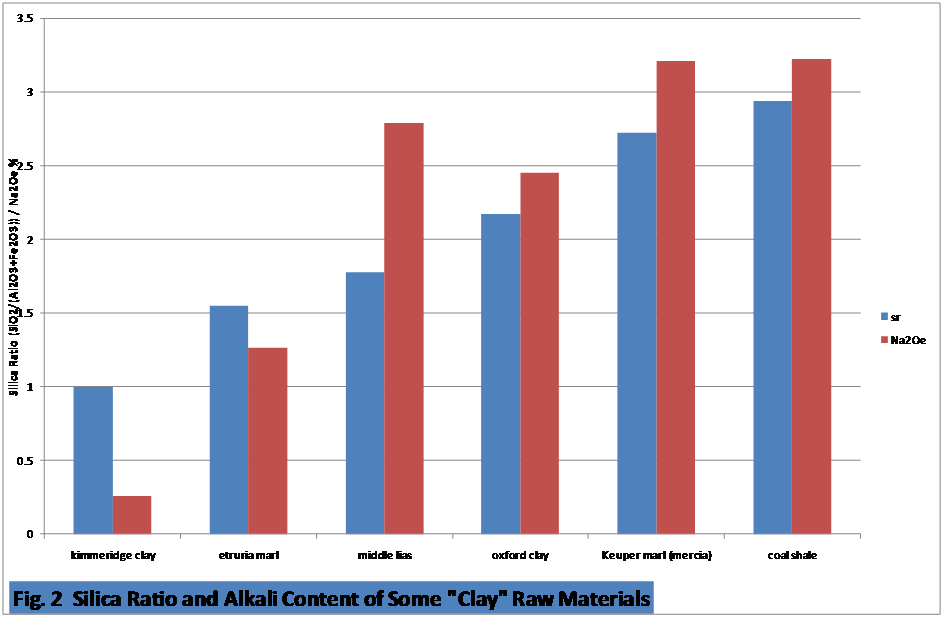 minerals in the shale can include a
proportion
of very finely divided silica,
especially if the igneous rock was
one
characterised as “acidic”, that is containing a high proportion of
quartz. This gives
the shale a high silica ratio
relative to a completely weathered clay.
The presence of very fine silica reduces the requirement
to add sand at
the raw mill to maintain the silica ratio of the raw feed and this, in
turn reduces
the milling required to achieve combination in the cement kiln.
minerals in the shale can include a
proportion
of very finely divided silica,
especially if the igneous rock was
one
characterised as “acidic”, that is containing a high proportion of
quartz. This gives
the shale a high silica ratio
relative to a completely weathered clay.
The presence of very fine silica reduces the requirement
to add sand at
the raw mill to maintain the silica ratio of the raw feed and this, in
turn reduces
the milling required to achieve combination in the cement kiln.
While
high silica
ratio clays are useful in reducing the sand input to the feed, there is
a
general relationship between the silica ratio of the clay and the
alkali
content as shown in Fig 2 for some typical raw materials. As more unweathered fine
sand is present in
the clay there may also be a higher proportion of unweathered alkali
feldspar. The
weathered products of
feldspars hold bases weakly and slightly acidic rainwater can remove
alkalis
from the clays by leaching, whereas the unweathered alkali feldspars
bring them
along to the kiln feed. Alkalis
can
provide problems of two kinds in cement manufacture.
They add to the recirculating load in the
kiln and can be complicit in causing blockages along with chlorides and
sulphate. In
addition there is a limit of
alkali permitted in cement due to the potential for
a disruptive reaction in concrete between
alkalis and some types of siliceous aggregate.
Many clay raw
materials also contain
significant proportions
of sulphur. The
igneous rocks from which
the clays derive do not usually contain more than trace amounts of
sulphur and
the presence in clays of both sulphates and sulphides is largely due to
changes
to the clay after it has been made from the parent rock. Clays which are deposited
in the sea may
assimilate sulphur from the sea water and those which are formed at
depth in
the oceans with little oxygen available contain sulphur concentrated
from the
decay of organisms.
As well as
sulphur these deposits may
contain significant
amounts of hydrocarbons and there are examples of clay raw materials in
many
parts of the world which contain an element of fuel when used in the
cement
kiln. Use of these
materials can,
however, lead to problems with emissions of sulphur oxides and, if
present as
sulphides, of carbon monoxide as the sulphur combines with available
oxygen in
the preheater tower.
Alternatives to
natural clays are
available in various
forms. The most
commonly used is probably
fly ash recovered form the combustion of fuel in coal fired power
stations. Compositions
of fly ash depend
on the nature of the shales associated with the coal used, but in
general they
are close to the clay raw materials which they replace.
|
|
|
|
|
|
|
|
|
|
Station
|
SiO2
|
Al2O3
|
Fe2O3
|
CaO
|
Na2Oe
|
LOI
|
|
Silica
ratio
|
|
|
|
|
|
|
|
|
|
|
|
Lynemouth.
|
48.20
|
30.10
|
8.10
|
2.10
|
1.40
|
4.30
|
|
1.26
|
|
Cottam
|
48.90
|
26.60
|
10.00
|
2.90
|
2.80
|
3.00
|
|
1.34
|
|
Drax
|
51.90
|
26.70
|
7.80
|
2.10
|
3.70
|
3.00
|
|
1.50
|
|
Ferrybridge
|
50.10
|
26.20
|
8.20
|
2.60
|
3.40
|
4.50
|
|
1.46
|
|
Rugeley
|
49.30
|
26.20
|
8.00
|
4.20
|
2.30
|
4.80
|
|
1.44
|
|
Ratcliffe
|
46.20
|
26.60
|
11.30
|
2.90
|
2.60
|
5.40
|
|
1.22
|
|
W
Burton
|
49.90
|
26.00
|
8.60
|
2.40
|
3.40
|
5.00
|
|
1.44
|
|
Longannet
|
51.50
|
29.40
|
5.50
|
3.00
|
1.00
|
|
|
1.48
|
|
|
|
|
|
|
|
|
|
|
Table
1 Typical
Compositions of UK Fly Ashes
|
|
|
|
An exception
to this is the silica
ratio of the fly ashes,
which tends to be lower than natural shales as can be seen by
comparison of the
compositions in Table 1 with those in Fig.2.
The fly ash silica ratios are significantly lower than the
coal shale
example in the Figure. This
is perhaps because
the relatively coarse silica particles in the shale are not carried to
the same
extent in the gas stream through the boiler and end up on the floor of
the
furnace in the bottom ash and not in the fly ash which was molten
in
the boiler itself.
With regard
to kiln feed processing
and clinker production a
valued property of clays and shales is the fine fundamental particle
size and
the relative ease of reducing the rock produced from the quarry to the
particle
size required for cement clinker burning.
In general fly ash used for clinker production is also of
a very fine
particle size. However,
the finest
portions of the ash produced from power stations also have a value as
cement
replacement materials if the carbon content is low enough to allow this. The economics of using fly
ashes as cement
raw material therefore can depend on the ability to use those fractions
which
would not be suitable for cement replacement and which would therefore
otherwise be landfilled at a cost to the producer.
The coarseness of the ash will have an effect
on the ability to achieve combination with minimum use of energy. Fig 3 demonstrates the
difference in the ease
of producing clinker of a given composition with ashes of different
fineness. The ash
described as “Part 1”
contains no more that 12% residue on a 45µ sieve
and no more than 7% LOI (essentially unburned
carbon). The
residue is the remaining
material left after the Part 1 has been mechanical separated from the
whole ash
and is therefore predominantly coarser than 45µ.
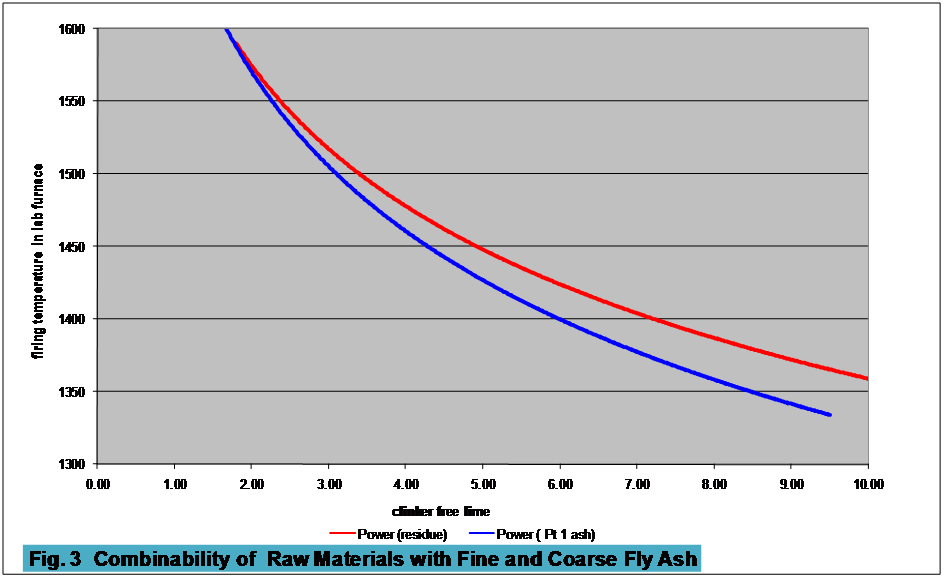
The shapes of
the combinability
curves are of interest, this
difference has been observed in other such comparisons.
At lower temperatures the finer ash is able
to reduce free limes more easily than the coarser ash, which would be a
predictable response. At
the higher
temperatures, however, the coarser ash is as well combined as the finer
ash. This
demonstrates an important difference with
the use of fly ash as opposed to natural clays or shales.
Clays are
sheet silicate crystal
structures with water
holding the sheets together. As
the
temperature in the cement kiln increases to about 700°C the first
effect on the
clay minerals is that they are dehydrated and begin at that stage to
decompose. This
leaves them very
susceptible to reaction with the surrounding materials and the first
intermediate products of cement clinker are formed very early. With fly ash this is not
the case. The ash
is in the form of glassy spheres with
little crystal structure to be disrupted and no inherent water to be
lost. Reactivity
with the other components of the
feed is very limited until the glass begins to melt at temperatures in
excess
of about 1000°C. The
chain of events to
produce the intermediate products is therefore different and, it would
seem
from the combinability studies, slower. The
larger fly ash particles evidently take
longer to melt and to become homogenised within the clinker, but when
this is
achieved combination can proceed as with the finer ashes.
Various other
waste products from
other industries can be
used to augment Limestone and clay as cement raw materials. Blastfurnace slag is used,
but the quantity
of magnesium in the slag frequently places a limit on the degree to
which this
is possible. Steel
slag is also valuable
but the iron content will always place a limit on this.
The advantages of materials which have
already been through a pyroprocessing stage are apparent in energy
conservation. It is
always essential,
however, to carry out thorough trials in the laboratory and on plant to
ensure
that the materials are compatible with the other raw materials and the
particular process.
A
Harrisson 2011
 minerals in the shale can include a
proportion
of very finely divided silica,
especially if the igneous rock was
one
characterised as “acidic”, that is containing a high proportion of
quartz. This gives
the shale a high silica ratio
relative to a completely weathered clay.
The presence of very fine silica reduces the requirement
to add sand at
the raw mill to maintain the silica ratio of the raw feed and this, in
turn reduces
the milling required to achieve combination in the cement kiln.
minerals in the shale can include a
proportion
of very finely divided silica,
especially if the igneous rock was
one
characterised as “acidic”, that is containing a high proportion of
quartz. This gives
the shale a high silica ratio
relative to a completely weathered clay.
The presence of very fine silica reduces the requirement
to add sand at
the raw mill to maintain the silica ratio of the raw feed and this, in
turn reduces
the milling required to achieve combination in the cement kiln.
