 My
geological background was invaluable as I started making laboratory
mixes and firing then to make new rocks which I then examined under the
microscope. In this way I learned about the observable
differences which small modifications to chemistry can make
for the potential quality of cement. Optimising the
raw
materials involves understanding their relative grindabilities as well
as combinabilities in various combinations. The
optimal mix
will not be the same in every market.
My
geological background was invaluable as I started making laboratory
mixes and firing then to make new rocks which I then examined under the
microscope. In this way I learned about the observable
differences which small modifications to chemistry can make
for the potential quality of cement. Optimising the
raw
materials involves understanding their relative grindabilities as well
as combinabilities in various combinations. The
optimal mix
will not be the same in every market.The clinker chemistry model as commonly used starts with the premise that the essential ingredients of cement clinker are the four oxides; SiO2, Al2O3, Fe2O3 and CaO which, when heated to approximately 1450°C in the cement kiln, form the four compounds; Ca3SiO5, Ca2SiO4, Ca3Al2O6 and Ca2AlFeO5. These are more generally known, using the cement industry notation, as C3S, C2S, C3A and C4AF, or else as alite, belite, aluminate and ferrite.
Using pure components and working under laboratory conditions, the fundamentals of how cement clinker is formed were elaborated and the ratios used today; Lime Saturation Factor (LSF), Silica Ratio (SR) and Alumina to Iron ratio (AF); with their implications for strength development, setting times and so on were established.
In practice of course it was always known that this was a simplification of the actual chemical mix in cement clinker and the compounds formed. The presence of the alkali oxides Na2O and K2O, of sulphur dioxide SO3 and of MgO were recognised as having an influence on the clinker formation process.
With increasing use of waste derived fuels and alternative raw materials, the significance of the minor components in cement clinker increases. Some traditional rules of thumb may need to be modified in the light of the new situation.
Liquid Formation
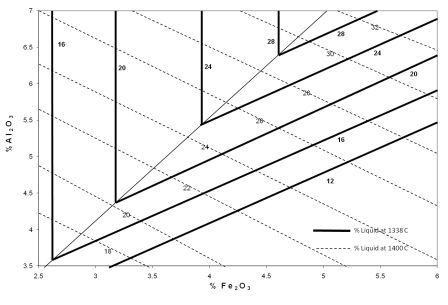
As
an example the optimum value for the AF
ratio, which will produce the maximum liquid at the lowest temperature,
was
identified as 1.38, as seen in the graphic reproduced as Fig.1. 
Figure 1. Liquid
Phase Formation and Liquid Composition
Within the
cement and concrete industry the potential
quality of a cement is usually judged in the first instance by the Lime
Saturation
Factor or by the C3S content as determined using
the Bogue
calculation. In
simple terms the more C3S
is present the better will be the 28 day strength.
The Bogue potential compound composition can
be calculated using a set of four simultaneous equations, each one
expressing
the quantity of SiO2(S), Al2O3(A),
Fe2O3(F)
or CaO(C) in each of the clinker compounds C3S, C2S,
C3A
and C4AF:

The solution to the equations will give the amount of S, A, F and C in C3S, C2S etc and therefore, in the pure system, the quantities of each compound.
In cement
clinker, however, pure compounds are not
formed. The minor
components in the raw
feed or from the fuels also become incorporated into the C3S,
C2S,
C3A and C4AF. The
modified silicate compounds C3S and C2S
are known by the
mineral names alite and belite respectively.
The compounds which are liquid in the kiln are generally
referred to as
the aluminate phase and the ferrite phase
or simply aluminate and
ferrite.
Table 1 shows typical real compositions of the clinker compounds,
compared to
the pure compounds’
compositions. 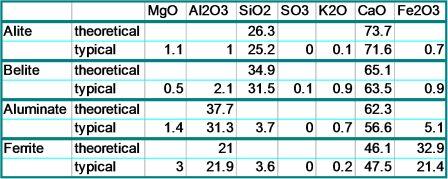
As long ago as the late nineteenth century, much of the original identification and characterisation of the clinker minerals was carried out using optical microscopical techniques. The relative quantities of the various visually distinct minerals were observed and the effects of different concentrations of each on clinker quality were noted. In an extension of the optical microscopic examinations, the use of the scanning electron microscope with x-ray microanalytical capability has allowed petrologists to obtain accurate analyses of the individual clinker minerals without the need for extensive extraction techniques. This procedure has allowed a large number of results to be produced. The typical analyses shown in Table 1 are averages of many such analyses from many sources. SEM analyses and interpretations of clinker are routinely carried out at WHD Microanalysis Consultants Ltd.
 pure compounds with those of more typical compositions or, if a
detailed
x-ray microanalysis of a particular clinker is available, with the
actual
composition of the minerals in that clinker.
Other techniques, including quantitative x-ray diffraction
analysis and
microscopic point counting are also available for assessing more
accurately the
actual compound composition of clinkers as opposed to the potential
compound
composition given by the Bogue calculation.
Table 2 shows a comparison of the results from each of
these methods on
a single clinker sample, compared to the Bogue calculation result.
pure compounds with those of more typical compositions or, if a
detailed
x-ray microanalysis of a particular clinker is available, with the
actual
composition of the minerals in that clinker.
Other techniques, including quantitative x-ray diffraction
analysis and
microscopic point counting are also available for assessing more
accurately the
actual compound composition of clinkers as opposed to the potential
compound
composition given by the Bogue calculation.
Table 2 shows a comparison of the results from each of
these methods on
a single clinker sample, compared to the Bogue calculation result.
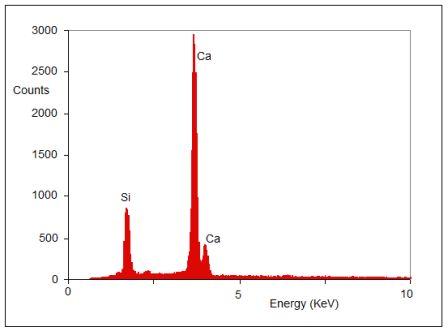
Figure 2 Example of x-ray microanalysis of calcium silicate.
|
|
Bogue Potential Compound Composition |
Modified Bogue Calculation |
Quantitative XRD |
Point Counting |
|
Alite |
57.7 |
65.6 |
68.8 |
61 |
|
Belite |
18.6 |
12.7 |
14.2 |
22 |
|
Aluminate |
9.7 |
6 |
|
17 |
|
Ferrite |
9.7 |
13.1 |
8.3 |
|
|
Free lime |
0.7 |
0.7 |
nd |
0.03 |
In every case the other methods give a higher value for the alite content of the clinker than the Bogue potential compound composition, due to the incorporation into alite of minor components.
There are obvious implications in these results for quality control and optimisation of the burning conditions in the kiln. The presence of appreciably higher levels of alite in the clinker than anticipated indicates that the effective LSF of the mix travelling through the burning zone is higher than the base chemistry would suggest. There is a strong link between the LSF of the kiln charge and burnability, with higher LSF mixes proving more difficult to burn (and therefore requiring more heat to achieve combination) than lower LSF mixes. If the clinker contains a variety of elements which are included in alite crystals but are not taken into account in the LSF calculation, then the desired combination will be more difficult to achieve. This may be manifested in difficulty reducing free limes to the target levels, in excessive heat consumption and possibly in increased emissions from the kiln. These will also be associated with reduced output.
As well as directly affecting the ratios used in clinker manufacture, the use of waste streams as alternative raw materials or fuels frequently introduces elements which have not traditionally been found in significant quantities in Portland Cement clinker. Many of these have implications for emissions and compliance with the appropriate environmental legislation. Some of them also have implications for the cement chemistry and the ease of combination of the clinker raw feed constituents.
Common examples of these elements would be phosphorous from MBM used as kiln fuel, magnesium from blastfurnace slags used as alternative raw materials and manganese from various waste streams derived from steel manufacture. In 2005 Earl Smith of Grace Construction provided an extensive list of possible minor elements and compounds which might be introduced from waste materials (ICR December 2005). A number of these elements are regarded as problematic due to their ability to form stable compounds or solid solutions with C2S, thereby preventing combination in the kiln and leading to high free lime contents and poor quality cement. This list would include strontium, boron and phosphorous. Sulphate could also be included because at moderate concentrations it has been reported as restricting or preventing the formation of C3S. In September 2003 an article in ICR described the influences of sulphate on C3S formation and also the rôle of fluorine in counteracting this. Mention was also made of the effect of phosphorous in limiting C3S formation.
Usually the economic case for using alternative materials containing these elements is overwhelming and in many cases a fall in quality is regarded as an acceptable price to pay. However, in many cases there are solutions available which will compensate for the quality loss when using waste streams. For example it has been well documented that the use of fluorine as a mineraliser in the case of high levels of sulphate can facilitate the lower temperature formation of C3S and produce a cement of higher quality than without these two components. Similarly it was demonstrated over 50 years ago that raw materials in Uganda containing high levels of phosphate could successfully be used to produce satisfactory quality cements if the fluorine which was also present in the raw materials was maintained in the clinker.
Preliminary laboratory trials by this author have also indicated that mixes containing levels of phosphorous, boron or strontium which, without other addition, prevent adequate combination can be satisfactorily combined if a proportion of fluorine can be introduced and maintained in the clinker.
Modern cement clinker making entails a number of restrictions which are to the benefit of the environment but with which previous generations have not needed to contend. The economic and environmental benefits of using waste streams which would otherwise be landfilled or incinerated are great. Their use does not mean necessarily, however, that quality of the product needs to suffer.
A Harrisson 2011